1 Introduction[1]
Like rice
and wheat, maize is an important cereal used globally for the production of
several products. Typically, maize belongs to the Poceace family; maize thrives in both tropical and temperate
regions of the world. Maize is an annual plant that produces grains that are
edible and as such have economic importance. Maize is produced in several
countries of the world notably, United States, China, Brazil, Mexico, India,
Argentina, Spain, Italy, Nigeria Australia etc.
Maize is
used for the production of several foods including fermented foods.
Abdulrahaman and Kolawole [1] listed 28 different food dishes that could be
produced from maize. Among the notable uses of maize is Ogi production (a fermented maize slurry), which is used especially
as weaning food for infants [2-5] as
well as a dietary staple for adults in West Africa especially for the
elderly and sick. Maize is also used for the production of Masa (a
sorghum-maize blend) [6]; Kokoro (a Nigerian indigenous fermented
maize snack) [7]; sekete (a fermented
maize beverage) [8]. In addition, Agidi can also be produced from
fermented maize slurry. Like ogi,
Agidi is an essential traditional cereal based food consumed in Africa that is
processed by natural fermentation [9, 10] of cereal mainly maize. Like Ogi,
Agidi is also consumed with soup, stew, akara, moimoi as light meal for the
sick [11, 12].
Maize
typically has health benefits. For instance, Abdulrahaman and Kolawole [1]
reported that maize has 6 medicinal values. Some of the major health benefits
of maize include as a rich source of vitamins and minerals; controls of
diabetes and hypertension; reduction in cholesterol absorption in the body and
risk of various cardiovascular diseases; anemia prevention; boosting of immune
system; maintenance of vision and healthy skin; lowering risk of colon cancer
and hemorrhoids and enhancement of bone strength [13]. Similarly, maize is rich in nutrients (i.e. dietary fiber,
carbohydrate, calories and low in protein), minerals (magnesium, phosphorus,
potassium and manganese) and vitamins (thiamin, vitamin C, folate and niacin)
[13]. However, during processing of
maize for ogi production, notably during sieving of milled fermented maize some
nutrients are lost. For instance Farinde [14] reported reduction in protein, ash,
fiber, carbohydrate, iron, zinc, magnesium, sodium and calcium content in
sieved ogi than in the un-sieved type.
During fermentation of maize for ogi production water is typically used
to ferment sieved maize. Authors have variously reported that initial
fermentation period of 1 – 3 days is ideal [3, 14-20]. On appearance, the maize gets swollen and the water
becomes turbid. On microbial presence, authors have variously reported that
microbial consortia of maize fermentation medium increases with density [2, 15,
18, 21, 22] and reduces in diversity as
fermentation progresses. Similarly, authors have reported that most of the
microbial diversity that participate in fermentation is mainly of the genera Lactobacillus,
Leuconostoc, Saccharomyces. Oyewole and Isah [23] listed genera of microbes
that play essential role in fermentation of traditional food to include Lactobacillus,
Lactococcus, Leuconostoc, Enterococcus, Streptococcus, Penicillium and Saccharomyces.
In a recent review by Izah
et al. [12], the authors reported that Saccharomyces cerevisiae,
Lactobacillus species and Aspergillus niger were the predominant
microbial isolates found in maize ogi fermentation medium.
Several studies have been
carried out with regards to microbial diversity of maize fermentation water
[15, 18, 21, 22, 24, 25]. Therefore,
this study focuses on the microbial characterization of four days maize
fermentation medium in Port Harcourt Metropolis.
2 Materials and Methods
2.1 Field Sampling
Dried yellow maize samples were purchased
from three maize sellers at Rumuomasi market, Port Harcourt, Nigeria. They were
packaged in sterile Ziploc bags and taken to the laboratory for fermentation.
2.2 Sample
preparation
In a sterile 1000 ml conical flask, the
maize samples were separately soaked using 950ml sterile water which was
aseptically added. The control was set
up (using sterile water without maize added). The cap of the conical flask was
loosely covered. 2ml of the ferment water samples from both media viz. the
flask containing maize and the one containing only sterile water was collected
after shaking for microbiological examination.
2.3 Identification of microbial isolates
Four media i.e. Nutrient agar (for total heterotrophic
bacteria counts), MacConkey Agar (for the enumeration of Enterobacteriaceae
family), Potato dextrose agar (for mould and Yeast), DeMan, Rogosa and Sharpe
Agar i.e. MRS Agar (for Lactobacillus). The media were prepared according to
the manufacturer’s instruction. The Pour plate method was used for TCFU m-1
determination employed. For bacteria (Enterobacteriaceace
family) 0.1 ml of the 10-fold serially diluted ferment water samples were
plated in the various media. For bacterial total viable counts, incubation was
for 24 – 48 hours at 37°C; for Mould and Yeast, incubation was for 3-4 days at
30°C; For Lactic acid bacteria incubation was at 37°C for 2-3 days under
anaerobic condition using MRS Agar containing 10mg/ml cycloheximide.
The bacteria isolates were subjected to biochemical
tests (viz: Gram’s reaction, citrate,
catalase, oxidase, Indole, coagulase, motility, methyl red) using the guides of
Cheesbrough [26] and Benson [27].
Thereafter, the resultant characteristics were compared with those of
known taxa using scheme of Cheesbrough [26] and Bergey’s Manual of
Determinative Bacteriology by Holt et al. [28]. Based on gram reaction, the
gram positive organisms were streaked in Mannitol Salt Agar plate and incubated
inverted at 37°C for 24 hours. The presence of yellowish pigments in Mannitol
Salt Agar indicates Staphylococcus aureus. Also, the pure cultures from
MacConkey agar were streaked in Levine’s eosin Methylene Blue (EMB) Agar and
incubated at 37º C for 24 hours. The presence of small nucleated colonies with
greenish metallic sheen indicates E. coli
[27, 29]. Similarly the lactic acid bacteria were further streaked in MRS agar
from where the colonial morphology and cellular characteristics for the various
colonies obtained were studied. Identification method for the lactic acid bacteria include macroscopic,
microscopic, and biochemical tests (such as gram reaction, catalase and sugar
fermentation i.e. lactose, sucrose, salicin, mannitol, sorbose, xylose and
growth on 4% sodium chloride following the method described by Oyarekua [30].
The resultant characteristics were compared with characteristics presented by
Oyarekua [30].
Both microscopic and
macroscopic techniques were employed for the identification of the moulds. The observation
of the mould isolates were compared with guide provided by Benson [27], while
the microscopic morphology was determined using
Lactophenol cotton blue stain as described by Pepper and Gerba [29] and Benson
[27]. The resultant microscopic characteristics were compared with the scheme
of Pepper and Gerba [29], Ellis et al. [31] and Benson [27].
The yeast isolates were streaked onto glucose yeast
agar and yeast malt agar under aerobic condition at 28ºC [32] from where
morphological and physiological tests were carried out. The result
characteristics were compared according to Barnett et al. [33], Kregger Van-Rij
[34], Ellis et al. [31] and Benson [27].
2.4
Statistical Analysis
The frequency of occurrence of microbial
isolates was computed and their type determined (i.e. as total heterotrophic
bacteria count/lactic acid bacteria counts and bacterial of the Enterobacteriaceace family) and fungi
(mould/yeasts). The charts were plotted using Microsoft excel.
3 Results and Discussion
The
microbial isolates of maize fermentation water for ogi production is presented
in Table 1. The bacterial isolates include Staphylococcus aureus, Escherichia coli, Enterobacter, Lactobacillus, Pseudomonas, Bacillus, Micrococcus and Corynebacteria species. While the mould
and yeast diversity included Aspergillus flavus, Aspergillus niger,
Penicillium, Rhizopus, Mucor, Fusarium,
Geotrichum species and Saccharomyces
cerevisiae. The distribution of microbes during the different fermentation of this
study showed similarity to the work of Adegbehingbe [2], who
reported that microbes such Corynebacteria,
Lactobacillus, Saccharomyces cerevisiae were found in the fermentation medium from the second
day upward. Similarly, the authors also reported that Staphylococcus aureus, Rhizopus nigricans and Candida
crusei were found in the fermentation medium on the first day and absent
from the second day upward, while Micrococcus lutens, Aspergillus niger
occurred from day 1 to day 2 but absent from day 3 upwards. The bacterial and
yeast/mould frequency of occurrence isolates are presented in Figures 2 and 3
respectively. Based on the frequency of occurrence the bacterial isolates
indicated; Staphylococcus aureus (34.9%) and Enterobacter sp
(3.3%) were highest and least at 0 hour. Lactobacillus sp (48.3%) and Enterobacter sp (2.3%) after 24 hours, Lactobacillus species (63.1%) and Pseudomonas, Bacillus and Micrococcus species (0.0%) occurred at 48
hours, Lactobacillus species (82.3 - 86.8%) and E.coli,
Pseudomonas, Bacillus and Micrococcus species (0 %) occurred at 72 to 96
hours of fermentation respectively (Figure 2). The frequency of occurrence of Lactobacillus species indicated that they
were increasing in number as fermentation progresses. Similarly, and Corynebacteria species progressed up to
48 hours before there was decline in the population. But other aerobic
mesophilic bacteria including E.coli, Pseudomonas, Bacillus, Enterobacter and Micrococcus species population
generally were in a decreasing trend. For yeast and mould isolates, the frequency of occurrence were highest
but least for Aspergillus niger (28.2%) and Saccharomyces
cerevisiae, Aspergillus flavus and Geotrichum species (0.0%) at 0 hour, Saccharomyces
cerevisiae (29.1%)
and Fusarium sp (5.2%) at 24
hours, Saccharomyces
cerevisiae (47.4%) and Fusarium, Rhizopus species and Aspergillus flavus (0.0%) at 48
hours, Saccharomyces
cerevisiae (80.8%)
and Geotrichum, Penicillium, Rhizopus, Fusarium, Mucor species and Aspergillus flavus (0.0 %) at 72 hours respectively (Figure 3). While only Saccharomyces cerevisiae were isolated at 96 hours
of fermentation. The trend in the frequency of occurrence in this study has
some similarity with the work of Akinleye et al. [22]. Enterobacter species and E.
coli are indicator organisms, thus their presence could stem from
contamination of maize by feacal material probably from materials used for
storage or even handling process.
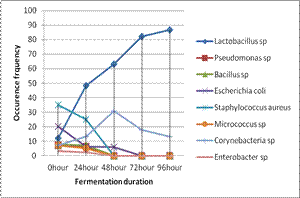
Figure 1: Occurrence
frequency of the bacteria isolates
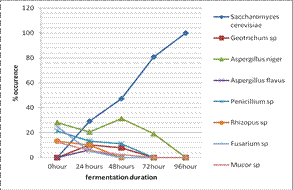
Figure 2:
Occurrence frequency of the yeasts/mould isolates